
Laboratory Stand “Training Vehicle with Built-in Fault Simulator” (Copy)
July 30, 2025
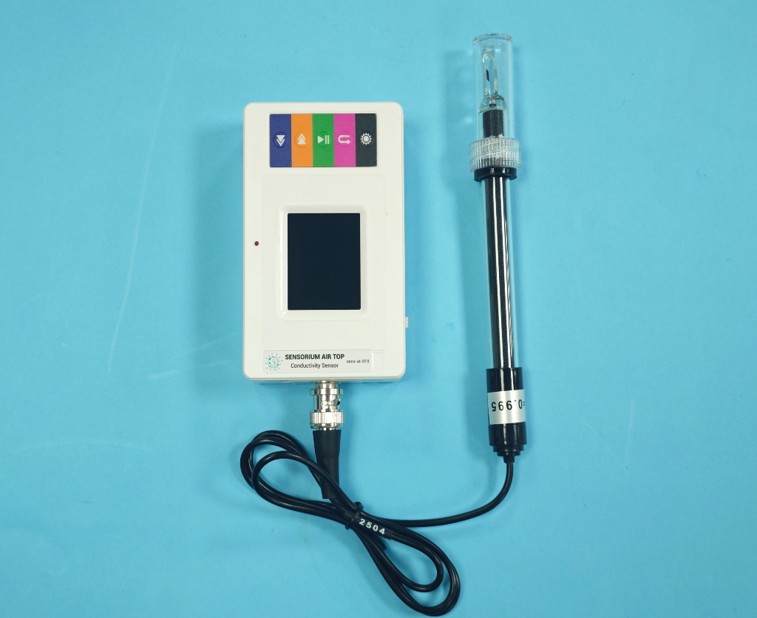
Wireless magnetic field sensor
August 1, 2025Wireless conductivity sensor
$556.00
Range: 0–20,000 μS/cm
Resolution: 1 μS/cm
I. Working Principle
The conductivity electrode has two platinum plates fixed on a built-in glass base, with their positions and distance preset. When the electrode is inserted into a liquid or conductive fluid, the sensor measures the solution’s conductivity by detecting the current between the two platinum plates. A potential difference is applied between the two platinum electrodes, and the resulting current is proportional to the solution’s conductivity. The sensor converts this current signal into a voltage signal, which is then transformed into a 0–5V output via the data collector’s analog-to-digital converter. The results are stored in the collector’s memory.
Since current in a solution is transmitted through ions, higher ion concentrations lead to stronger conductivity. Therefore, the conductivity sensor can also be used to measure ion concentration in solutions.
II. Specifications
Range: 0–20,000 μS/cm
Resolution: 1 μS/cm
III. Structure and Features
1. Equipped with a 1.8-inch color LCD screen.
2. Configured with five functional buttons for simple and convenient operation.
3. Supports high-speed USB data transfer for fast communication with data collectors.
4. Enables wireless communication with experimental terminals.
5. Features a built-in large-capacity removable battery.
6. Includes a snap-on sensor interface for compatibility with standard sensors in combined experiments.
7. Provides a fixed mounting position for integration with traditional equipment on an iron stand.
8. Compatible with iOS, Android, and Windows systems.
IV. Typical Applications
The conductivity sensor can be used in the field or laboratory to measure solution conductivity or total ion concentration. While it cannot identify specific ion types, it quickly determines the total ion concentration. Typical applications include studying the conductivity of different solutions, environmental research, monitoring water purity, and investigating the effects of chemical coagulation on conductivity, among others.
V. Note
This product is suitable for educational purposes only and not for industrial, medical, research, or commercial applications.
VI. Usage Tips
1. Rinse the sensor electrode tip with distilled water before use.
2. When inserting the sensor tip into the sample solution, ensure the electrode is fully submerged.
3. After use, rinse the electrode with distilled water and dry it with a paper towel or lab cloth before storage. Avoid damaging the electrode’s inner surface during drying.
4. Fats, oils, fingerprints, and other residues on the electrode may cause inaccurate measurements and unstable responses.
5. If the sensor probe is contaminated, soak it in water with mild detergent for 15 minutes, followed by immersion in a diluted acid solution (preferably 0.1 M HCl or 0.5 M acetic acid) for another 15 minutes, then rinse thoroughly with distilled water.
6. For storage, it is best to soak the probe in deionized water. If stored dry, soak it in deionized water for 5–10 minutes before use.
7. The conductivity sensor probe is made of glass and is fragile—handle with care.
8. Operating temperature range for the conductivity sensor probe: 0–80°C.
9. Response time: Achieves 98% of readings within 10 seconds.
10. Service life of the conductivity probe: 1 year.
11. pH and temperature sensors should not be connected to the same data collector as the conductivity sensor or placed in the same solution simultaneously (because the conductivity sensor generates an output signal in the solution, which may interfere with other sensors).
12. If simultaneous use with the above sensors is necessary, the following methods are available: To obtain readings for both conductivity and pH or conductivity and temperature, connect the sensors to two separate data collectors.




Reviews
There are no reviews yet.